- Calculation Of Average Atomic Mass Of Chlorine
- Average Atomic Mass Of Chlorine
- What Is The Average Atomic Mass Of Chlorine
- Average Atomic Mass Of Chlorine In Amu
2.4 Atomic Weights
Atoms are small pieces of matter, so they have mass. As noted in Section 2.1, a key postulate of Dalton's atomic theory is that mass is conserved during chemical reactions. Much of what we know about chemical reactions and the behavior of substances, therefore, has been derived by accurate measurements of the masses of atoms and molecules (and macroscopic collections of atoms and molecules) that are undergoing change. Chances are that you are already using mass measurements in the laboratory portion of your course in order to monitor changes that occur in chemical reactions. In this section we will discuss the mass scale that is used for atoms and introduce the concept of atomic weights. In Section 3.3 we will extend these concepts to show how these atomic masses are used to determine the masses of compounds and molecular weights. Cyberghost mac os x download.
Calculation Of Average Atomic Mass Of Chlorine

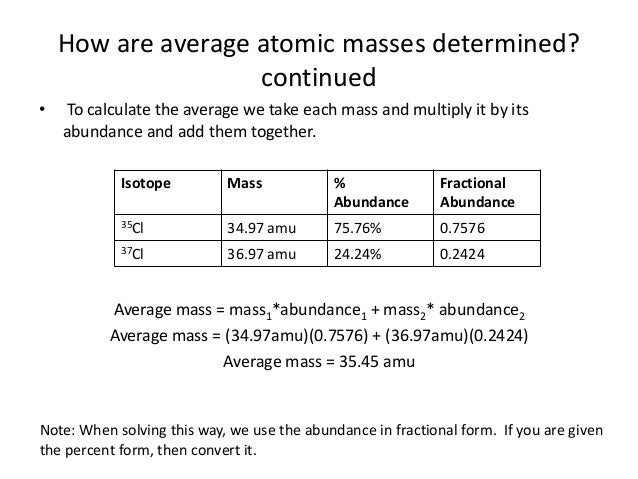
The atomic mass of 35,17CL (75.53%) and 37,17Cl (24.47%) are 34.968 amu and 36.956 amu, respectively. Calculate the average atomic mass of chlorine. The percentages in parentheses denote the. Chlorine-35 atoms have a mass of 34.96885 amu. All other chlorine atoms are chlorine-37 and these have a mass of 36.96590. Calculate the adverage atomic mass of chlorine. My answer: mass of. The average atomic mass of chlorine = (75.77 × 34.9689 + 24.23 × 36.9659)/100 = 35.4527 u Please send your queries to ncerthelp@gmail.com you can aslo visit our facebook page to get quick help. Link of our facebook page is given in sidebar.
Atomic Mass of Chlorine. Atomic mass of Chlorine is 35.453 u. The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. We use the most common isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages.
The Atomic Mass Scale
Although scientists of the nineteenth century knew nothing about subatomic particles, they were aware that atoms of different elements have different masses. They found, for example, that each 100.0 g of water contains 11.1 g of hydrogen and 88.9 g of oxygen. Thus, water contains times as much oxygen, by mass, as hydrogen. Once scientists understood that water contains two hydrogen atoms for each oxygen, they concluded that an oxygen atom must have times as much mass as a hydrogen atom. Hydrogen, the lightest atom, was arbitrarily assigned a relative mass of 1 (no units), and atomic masses of other elements were at first determined relative to this value. Thus, oxygen was assigned an atomic mass of 16.
Average Atomic Mass Of Chlorine
Today we can determine the masses of individual atoms with a high degree of accuracy. For example, we know that the atom has a mass of g and the atom has a mass of g. As we noted in Section 2.3, it is convenient to use the atomic mass unit (amu) when dealing with these extremely small masses:
What Is The Average Atomic Mass Of Chlorine
Average Atomic Mass Of Chlorine In Amu
The amu is presently defined by assigning a mass of exactly 12 amu to an atom of the isotope of carbon. In these units the mass of the nuclide is 1.0078 amu and that of the nuclide is 15.9949 amu.
